Problems in industry due to water hardness
- Posted by: arvengtraining
- Category: Process Engineering

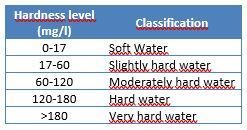
The presence of calcium (Ca2+) and magnesium (Mg2+) ions in a water supply is commonly known as “hardness.” The degree of hardness is classified as follows:
The main problem associated with hardness is scale and deposits formation. Even levels as low as 5 to 8 mg/L are too extreme for many uses (i.e. as boiler feed water).
Hardness, and in particular calcium, tend to precipitate coupling with anions like carbonate, phosphate or sulphate. Calcium and magnesium carbonates solubility is in inverse proportion to temperature, which means that as higher the temperature, the lower the solubility is.
Hardness in water is the main source of scale formation in heat transfer equipment, boilers and pipelines:
- It will cause buildup of scale in pipes causing reduced flow and pressure drop increase, then causing greater pumping cost associated, and eventually causing pipes to need to be internally cleaned or replaced.
- Hard water in cooling towers can reduce the effectiveness of heat transfer, causing increased running costs. It can also cause corrosion in the towers, leading to costly repairs or filling replacement of cooling towers.
- In boilers, once scale begins to form on tube surfaces, it creates another layer of material through which heat must pass to get to the water. Two main problems occur due to the insulative effect of the scale material: under deposit corrosion and overheating of the boiler tube metal (creating “hot spots”), leading to water tubes failure and stress corrosion cracking, pushing up operational costs. It also causes the need for more costly maintenance and repairs and will reduce the lifespan of the boiler.
Hence, poor quality of water used in industry is one of the major causes of economic loss due to equipment mechanical failure and maintenance problems. All the above ultimately lead to unplanned turnarounds to make repairs and chemical/mechanical cleanings. Chemical cleaning represents a less expensive option than mechanical cleaning (e.g. hydro-blast). Nonetheless, while the latter typically complete removes the fouling deposits, the effectiveness of chemical cleaning depends on many factors (choice of chemical, deposit composition and ageing, etc.) and often leaves the surface only partially clean.

Figure 1. Scale deposits in a boiler
The use of mineral and/or organic conditioning products (such as scale and corrosion inhibitors), can be used to avoid deposits, scaling and/or corrosion and, therefore, to reduce either the cost of make-up water treatment. Nevertheless, the most effective way to prevent scaling and related problems is to prevent impurities like hardness from entering in the system by doing a proper water treatment by filtration, ion exchange, reverse osmosis, etc.
If you want to know more:



 WhatsApp
WhatsApp