Defining raw water needs as well as potential raw water source, is the starting point of work to define the water treatment methods in Industry applications. Water treatment processes will be as much intensive as it is the water quality required for the process itself, being the purity of water used to feed high pressure boilers the most stringent quality condition.
Industries can use different raw water sources, mainly surface waters and ground waters. Some pollutants that could be present in those type of waters include atmospheric gases, minerals, organic material and microorganisms. Those contaminants could be found dissolved in the water or as suspended matter.
Groundwater is essentially rain water that percolates through the soil until the soil is saturated. As rain falls through the air, it absorbs carbon dioxide and the resulting acid then dissolves minerals contained in the soil it contacts. Ground water is well filtered as it percolates through the soil and is usually free of suspended matter and color. However, since groundwater composition is related to the chemistry of the geologic formations through which the water passes, most of groundwaters have calcium, magnesium and alkalinity typical of limestone-based formations. Other formations contribute with iron, sulfates or chlorides, all of those giving a typical “hardness” characteristic to the underground water.
Surface waters contain less dissolved minerals compared to underground water, but instead, they have higher organic pollutants content as well as non-dissolved particles, since surface waters are in normally in contact with surface debris and flora.
ION EXCHANGE DEMINERALIZATION is one of the most well-known processes used in water treatment for dissolved solids reduction, for both for groundwaters and surface waters. Ion exchange is the traditional method for softening and for the removal of dissolved solids (ions) in high purity water treatment applications, where those salts could generate deposits, especially in heat exchangers, then resulting in heat transfer reduction potentially leading to mechanical and thermal stress failure.
Ion exchange processes use ion exchange resins. Those are synthetic copolymers or acrylic polymers insoluble in water. As waster passes through the resin, ion exchange occurs, removing targeted ions from the water and replacing them with more desirable ions. Ion exchange resins structure includes acidic radicals or basic radicals, where mobile ions are located. Those mobile ions are exchanged with cations and anions present in water coming from dissolved mineral salts. Then, cation resin exchanges desirable cations to the water, while anion resin exchanges desirable anions to the water, as schematically indicated in Figure 1.
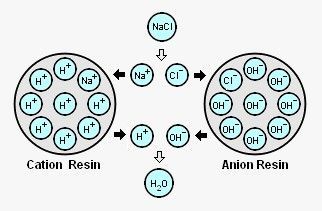
Figure 1: Ion exchange
There are two types of ion exchange resins:
- Cation resins: used for removing cations dissolved in water such as Na+, Ca2+, Mg2+, Fe3+, etc., then displacing the mobile ion H+ or Na+ from resin structure.
- Anion resins: used for removing anions dissolved in water such Cl– , SO42-, CO32-, NO3–, etc, then displacing the mobile ion OH– o Cl– from resin structure. Anion resins can remove also silica from water, since silica acts as a weak anion.
Ion exchange resins present “affinity”, as a reflection of the relative selectivity for ions. Then, affinity is strongest with higher ion valence and higher atomic number. In simple terms, cation resin prefers calcium ion to sodium. It is clear then, that ion exchange is a batch process and, consequently, after certain time, when all of the ion exchange beads in the resin bed have exchanged all of their mobile ions for process water ions, the resin bed is said to be “exhausted” and must be regenerated.
A clear symptom of resin exhaustion is a conductivity increase in water outlet. That happens when occurs the namely “ion leak”, which is the moment when the resin cannot retain those ions which are last in the selectivity hierarchy and those ions start to leak to service water. At this point, the resin must be regenerated. Resin regeneration is accomplished by using an acid or base concentrated solution through the resin bed. For cation resins a sulphuric acid (H2SO4) solution or hydrochloric acid (HCl) solution is normally used, although the later presents more trouble in terms of metallic corrosion. For anion resins a caustic solution (NaOH) is used.
It is important to note that the regenerant solution may change as a function on the acid or basic nature of the resin structure. Also, the regeneration process could be done in different manners (countercurrent, co-current, etc).
The ion exchange process is then reversible, meaning that the resin bed can be recharged once exhausted, getting ready to start over the deionization process again. The results on the ion exchange demineralization is a reduction of 95-99-9 % of dissolved solids in water to low part per million (ppm) concentrations in high purity water treatment applications, then competing with reverse osmosis.
If you want to know more:




 WhatsApp
WhatsApp